Tissue models for evaluating prolonged PFAS exposures
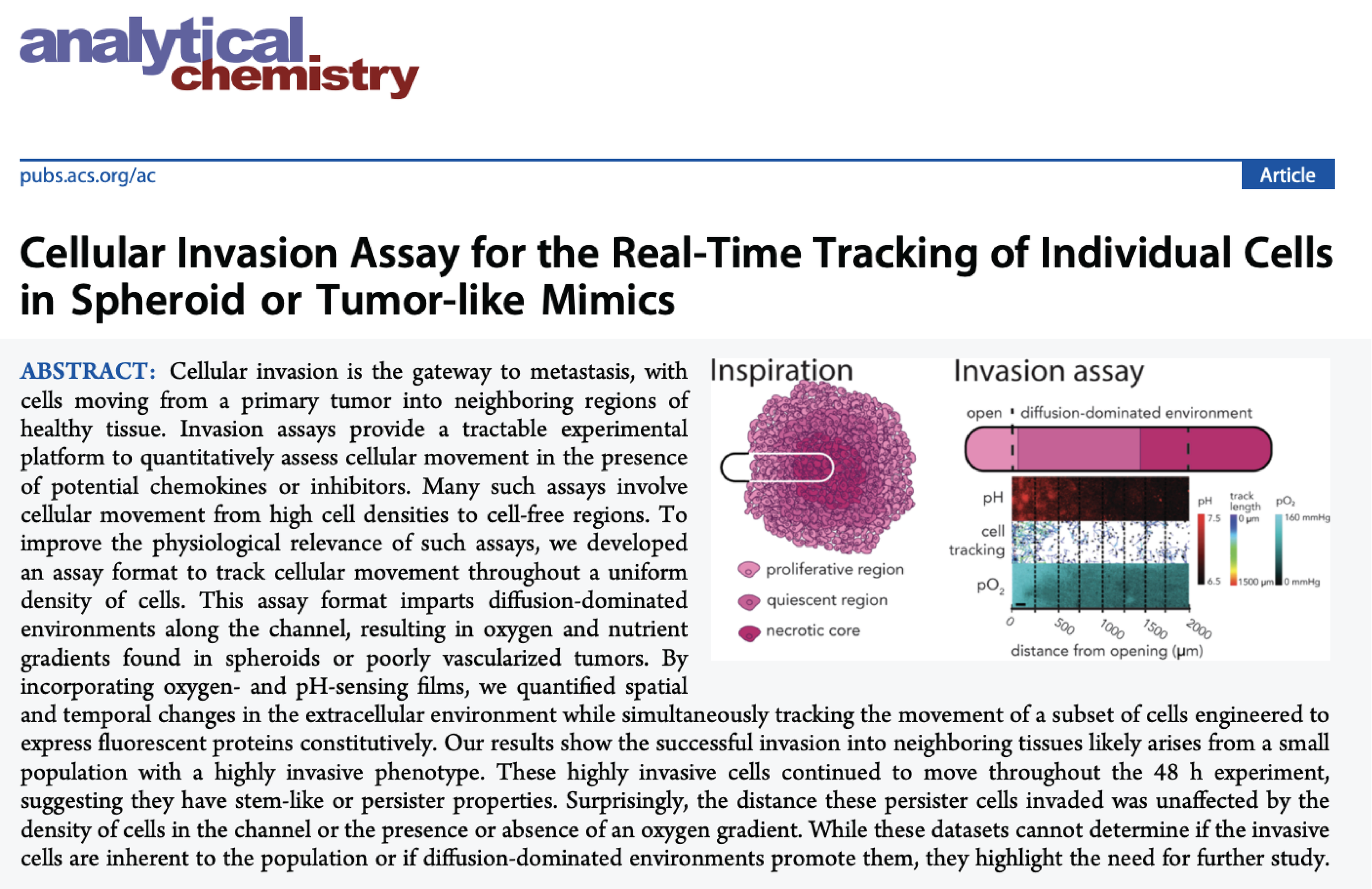
Paper-based cell invasion assay development
We developed several invasion assay formats that rely on paper-based scaffolds, capable of supporting cell-laden gels. These assays can quantify cellular movement under different experimental conditions, and provide quantitative readouts in the form of: 1) the number of cells that invaded from a single scaffold, 2) the number, distance, and direction cells invaded in the presence of extracellular gradients, and 3) populations of cells segmented by the distance they invaded.
We continue to develop:
Invasion assays in which individual cells can be tracked and then extracted to determine biomarker signatures that arise for highly invasive phenotypes.
Assay formats that allow for correlative studies of distance invaded and drug-resistance within single cells.
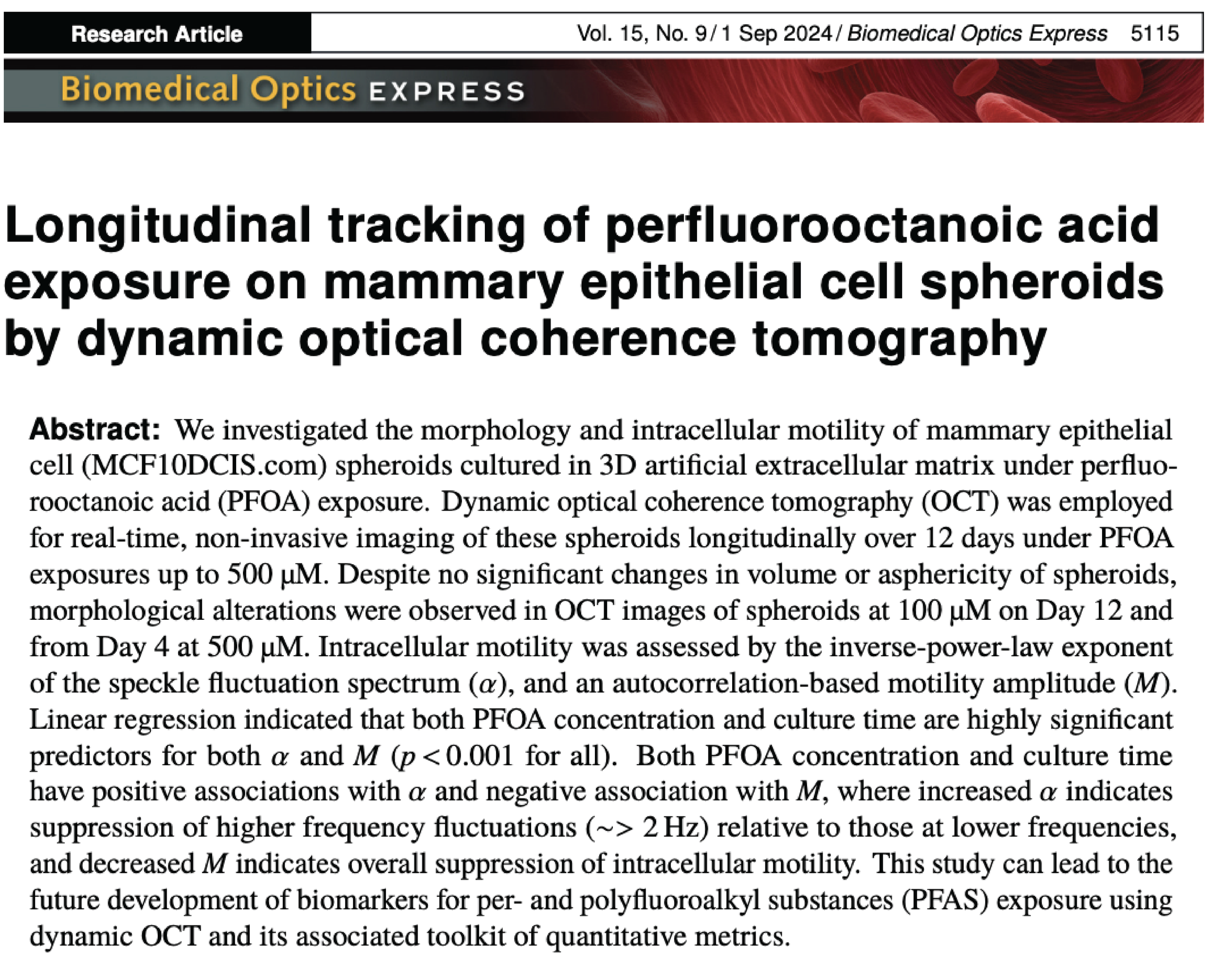
PFAS exposure effects on steatosis
The liver is site of xenobiotic metabolism and the production of albumin. PFAS are known to increase oxidative stress in hepatocytes, altering the lipid synthesis and beta-oxidation. We previously developed a lipidomic workflow that focuses on identifying lipid species that undergo the largest changes between a control and experimental group. We are applying this approach to evaluate changes in lipid storage and synthesis. We are currently:
Quantifying changes in AhR-directed expression of xenobiotic metabolizing enzymes by PFAS exposures.
Developing a high-content imaging and LC-MS/MS lipidomic workflow to quantify altered lipidomic regulation in hepatocytes maintained in physiologically relevant organ structures and microenvironments.
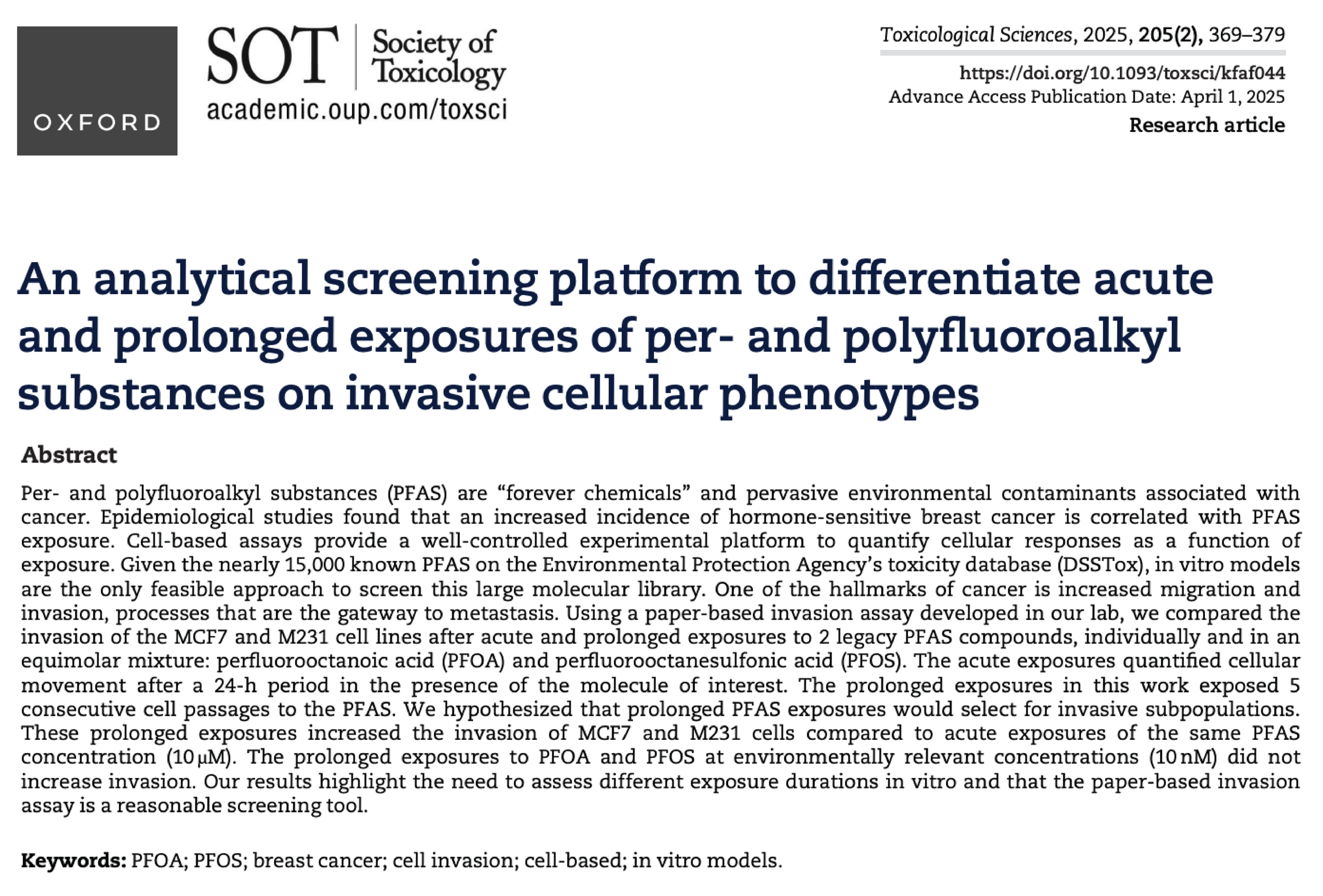
PFAS exposure effects on breast cancer progression
Many environmental exposures to toxins occur over prolonged periods at concentrations that are sub-nM (or lower) in concentration. Experimental screening procedures to identify potential toxins evaluate compounds at mM concentrations for periods of less than 48h, a practice that disregards accumulated cellular mutations or evolved phenotypes. Motivated by our recent findings that prolonged (but not acute) exposures to PFAS promote cellular invasion, we are:
Evaluating increasingly complex combinations of PFAS, representing environmentally relevant mixtures and concentrations, to quantify their effects on altered (increased or decreased) invasiveness.
Elucidating the molecular mechanisms by which PFAS alter cellular invasiveness.