Quantitative NAMs for assessing hepatotoxicity of new chemical entities
Liver-focused millifluidic device fabrication
The liver is responsible for metabolizing the majority of xenobiotics that enter the body, a process orchestrated through the tight regulation of phase I and phase II (conjugating) enzymes. Devices that can mimic key aspects of the liver microenvironment can better predict drug toxicity and drug-drug interactions than the monolayer cultures currently relied upon. We are developing millifluidic devices that incorporate the appropriate architecture, cell composition, and continuous perfusion found within the liver sinusoid. In particular, we are:
Developing co-culture models of hepatocytes and non-parenchymal cells, supported in paper-based scaffolds.
Constructing continuous flow devices that can introduce a flowing medium across endothelial cells in a sinusoid-like (porous) configuration.
Predictive modeling of drug-drug interactions
Pharmacokinetic and pharmacodynamic studies inform predictions of likely drug-drug interactions (DDIs). These predictions can prove challenging for first-in-class drugs, and in patients who take many medications (so-called polypharmacy). Adverse outcomes from DDIs are further complicated by the reliance of many patients on dietary supplements, which can range from a multivitamin to nutraceuticals like St. John’s-wort. We are developing high-throughput screening methods to assess potential DDIs in 3D liver-like cultures, with a focus on metabolomic characterization of inputs (nutraceutical formulations) and outputs (newly generated metabolites) to inform predictive computational models.
Hepatocyte post-differentiation
The many tasks carried out by hepatocytes (e.g., xenobiotic metabolism, albumin production, lipid biosynthesis/ metabolism, urea generation) are distributed across different zones along the sinusoid. This division of labor is dictated by chemical signals, including those from oxygen. We measure the effects of physiologically relevant oxygen tensions (as well as exposure to key signaling molecules) on hepatocyte functions and their underlying transcriptional regulation. With different experimental setups and newly generated tools, we:
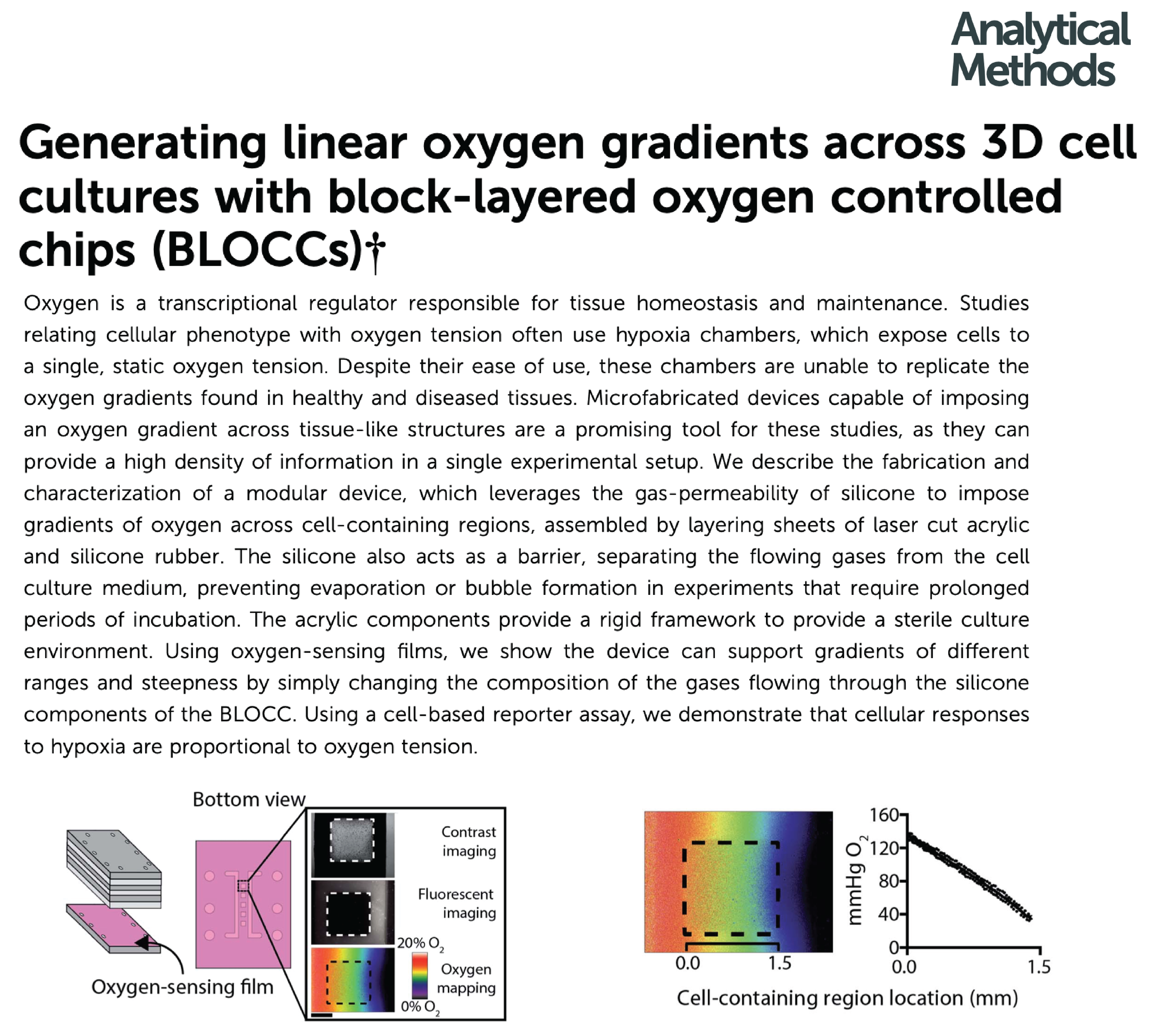
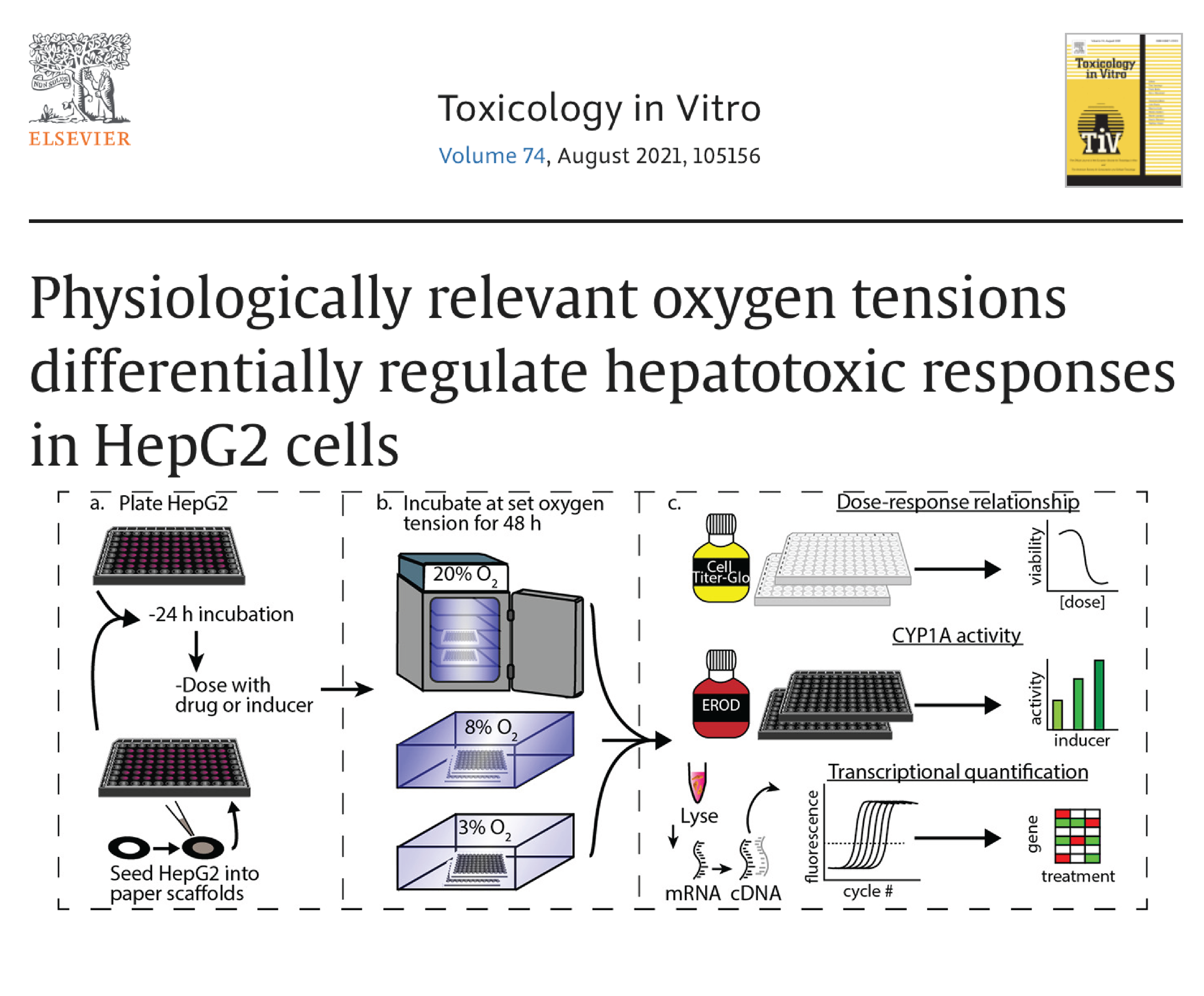
Measure altered expression and activity of phase I and II xenobiotic metabolizing enzymes in defined oxygen gradients, imparted in a traditional hypoxia chamber or through BLOCC devices.
Determine the polarization that occurs in 3D environments under different oxygen tensions, in combination with key effectors of the Wnt/b-catenin signaling pathway.
Evaluate plasticity and the time scale under which hepatocytes can remodel their function..